Art-ranking activity a cardiac contractile cell action potential delves into the intricate electrical properties and regulatory mechanisms that govern the rhythmic beating of the heart. This exploration unveils the fundamental principles underlying cardiac function and provides a comprehensive understanding of the cardiac action potential, its clinical significance, and advanced techniques employed to study it.
The electrical properties of cardiac contractile cells, including the resting membrane potential and the role of ion channels in generating the action potential, lay the groundwork for understanding the heart’s electrical activity. The phases of the cardiac action potential and their ionic basis provide insights into the precise sequence of events that drive cardiac contraction.
Electrical Properties of Cardiac Contractile Cells: Art-ranking Activity A Cardiac Contractile Cell Action Potential
Cardiac contractile cells, also known as cardiomyocytes, exhibit unique electrical properties that enable them to contract and pump blood effectively. The resting membrane potential of these cells is typically around -85 mV, which is maintained by the interplay of various ion channels and pumps.
Role of Ion Channels in Action Potential Generation
The generation of the cardiac action potential is a complex process involving the opening and closing of specific ion channels. The influx of sodium ions through voltage-gated sodium channels (Na v1.5) triggers the upstroke of the action potential. This is followed by the opening of L-type calcium channels (Ca v1.2), which allows calcium ions to enter the cell and contribute to the plateau phase of the action potential.
Phases of the Cardiac Action Potential and Their Ionic Basis
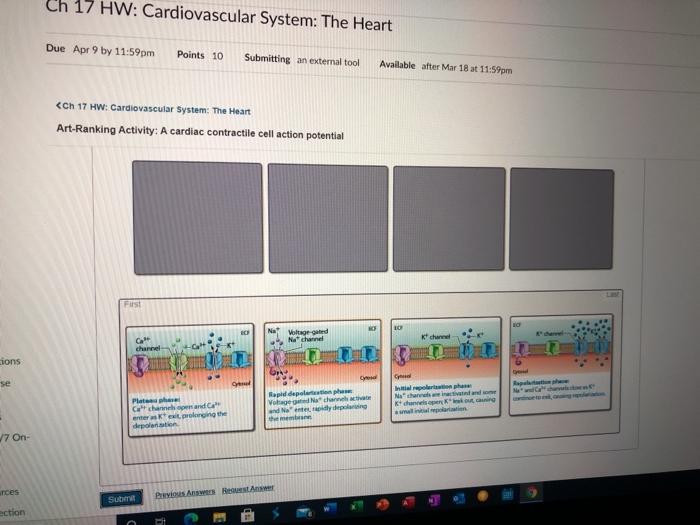
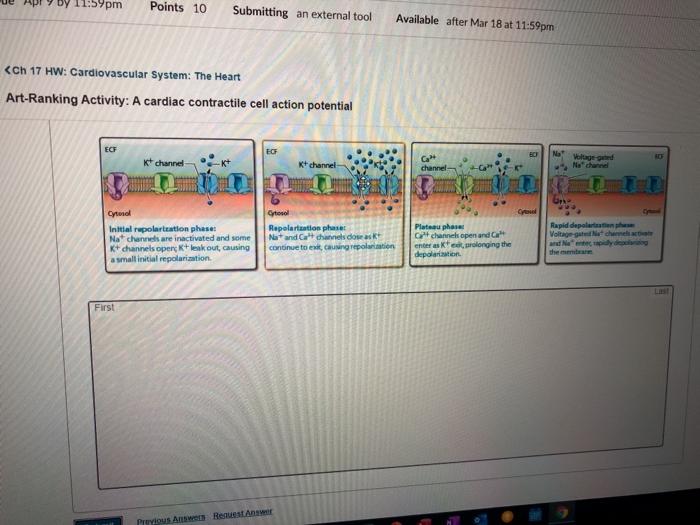
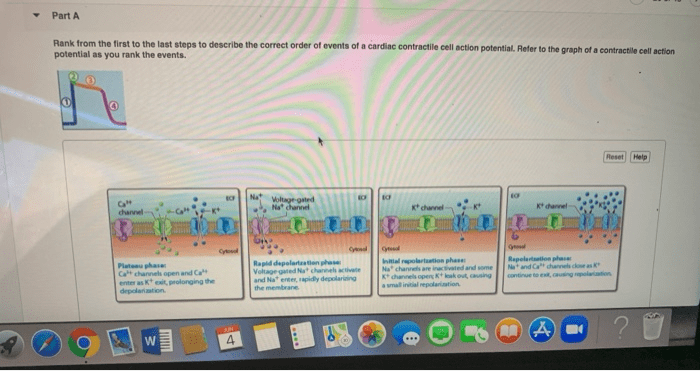
The cardiac action potential can be divided into five distinct phases:
- Phase 0:Rapid depolarization due to Na +influx through Na v1.5 channels.
- Phase 1:Transient repolarization due to inactivation of Na v1.5 channels and opening of transient outward potassium channels (I to).
- Phase 2:Plateau phase due to the sustained influx of Ca 2+through Ca v1.2 channels and the efflux of K +through voltage-gated potassium channels (K v4.3).
- Phase 3:Rapid repolarization due to inactivation of Ca v1.2 channels and increased K +efflux through K v4.3 and other K +channels.
- Phase 4:Resting membrane potential due to the maintenance of K +efflux and the activity of the Na +/K +ATPase pump.
Regulation of Cardiac Contractility
The amplitude and duration of the cardiac action potential are tightly regulated to ensure optimal cardiac contractility. This regulation involves various mechanisms:
Mechanisms Regulating Action Potential Amplitude
The amplitude of the action potential is primarily determined by the influx of Na +and Ca 2+ions during phases 0 and 2, respectively. Factors that influence these ion currents, such as changes in membrane potential, extracellular ion concentrations, and the availability of ion channels, can modulate the action potential amplitude.
Mechanisms Regulating Action Potential Duration
The duration of the action potential is mainly controlled by the balance between inward Ca 2+currents and outward K +currents. Prolonged action potentials can result from increased Ca 2+influx or reduced K +efflux, while shortened action potentials can occur due to decreased Ca 2+influx or increased K +efflux.
Role of Autonomic Nervous System and Hormones, Art-ranking activity a cardiac contractile cell action potential
The autonomic nervous system and hormones play a significant role in modulating cardiac contractility by influencing the action potential. Sympathetic stimulation increases heart rate and contractility by increasing Ca 2+influx and reducing K +efflux, while parasympathetic stimulation has the opposite effects.
Excitation-Contraction Coupling
Excitation-contraction coupling refers to the process by which the electrical signal of the action potential is translated into mechanical contraction. In cardiac cells, the influx of Ca 2+during the action potential triggers the release of Ca 2+from the sarcoplasmic reticulum (SR), which binds to troponin C and initiates muscle contraction.
Clinical Significance of Action Potential Abnormalities
Abnormalities in the cardiac action potential can lead to various arrhythmias, which are abnormal heart rhythms. These arrhythmias can range from benign to life-threatening and require appropriate diagnosis and treatment.
Common Arrhythmias Resulting from Action Potential Abnormalities
Common arrhythmias that arise from action potential abnormalities include:
- Atrial fibrillation:Characterized by rapid and irregular atrial activity due to multiple re-entry circuits within the atria.
- Ventricular tachycardia:A rapid heart rate originating from the ventricles, often due to increased automaticity or re-entry.
- Long QT syndrome:A genetic disorder characterized by prolonged action potential duration, which can lead to torsades de pointes, a potentially fatal arrhythmia.
Diagnostic and Therapeutic Approaches
Diagnosis of arrhythmias involves electrocardiography (ECG) and other electrophysiological studies to assess the electrical activity of the heart. Treatment options may include medications to modulate ion channels, catheter ablation to eliminate abnormal electrical pathways, and implantable devices such as pacemakers or defibrillators to regulate heart rate and rhythm.
Impact of Drugs and Interventions
Various drugs and interventions can affect the cardiac action potential. For example, antiarrhythmic drugs can block specific ion channels to prevent or terminate arrhythmias. Catheter ablation involves the destruction of abnormal electrical pathways, while implantable devices can provide electrical stimulation or defibrillation to correct heart rhythm.
Advanced Techniques for Studying Cardiac Action Potentials
Advanced techniques have been developed to study cardiac action potentials in greater detail and to understand their role in arrhythmogenesis.
Patch-Clamp Technique
The patch-clamp technique is a powerful electrophysiological tool that allows researchers to record ion currents from single ion channels in isolated cells. This technique has provided invaluable insights into the molecular basis of cardiac action potentials and arrhythmias.
Optical Mapping and Other Imaging Techniques
Optical mapping and other imaging techniques, such as voltage-sensitive dyes and calcium indicators, enable the visualization of action potentials across the entire heart or specific regions. These techniques have been instrumental in understanding the mechanisms of arrhythmia initiation and propagation.
Computational Modeling
Computational modeling plays a crucial role in simulating and predicting cardiac action potentials and arrhythmias. These models incorporate detailed mathematical representations of ion channel kinetics, cellular electrophysiology, and tissue architecture to study the complex interactions that govern cardiac electrical activity.
Common Queries
What is the resting membrane potential of cardiac contractile cells?
The resting membrane potential of cardiac contractile cells is approximately -85 mV, which is maintained by the balance of inward and outward ion currents.
How do ion channels contribute to the generation of the cardiac action potential?
Ion channels, such as sodium, potassium, and calcium channels, allow the selective movement of ions across the cell membrane, creating changes in membrane potential that underlie the phases of the cardiac action potential.
What is excitation-contraction coupling?
Excitation-contraction coupling refers to the process by which the electrical signal of the action potential is translated into a mechanical contraction of the cardiac muscle.